HCOOCH CH₂ H₂O Explained : Simple 2025 Guide
Chemistry often has short formulas that look hard to understand. One of them is hcooch ch2 h2o Explained . Many students and readers search for this to know what it means Pertadad
In very simple words:
-
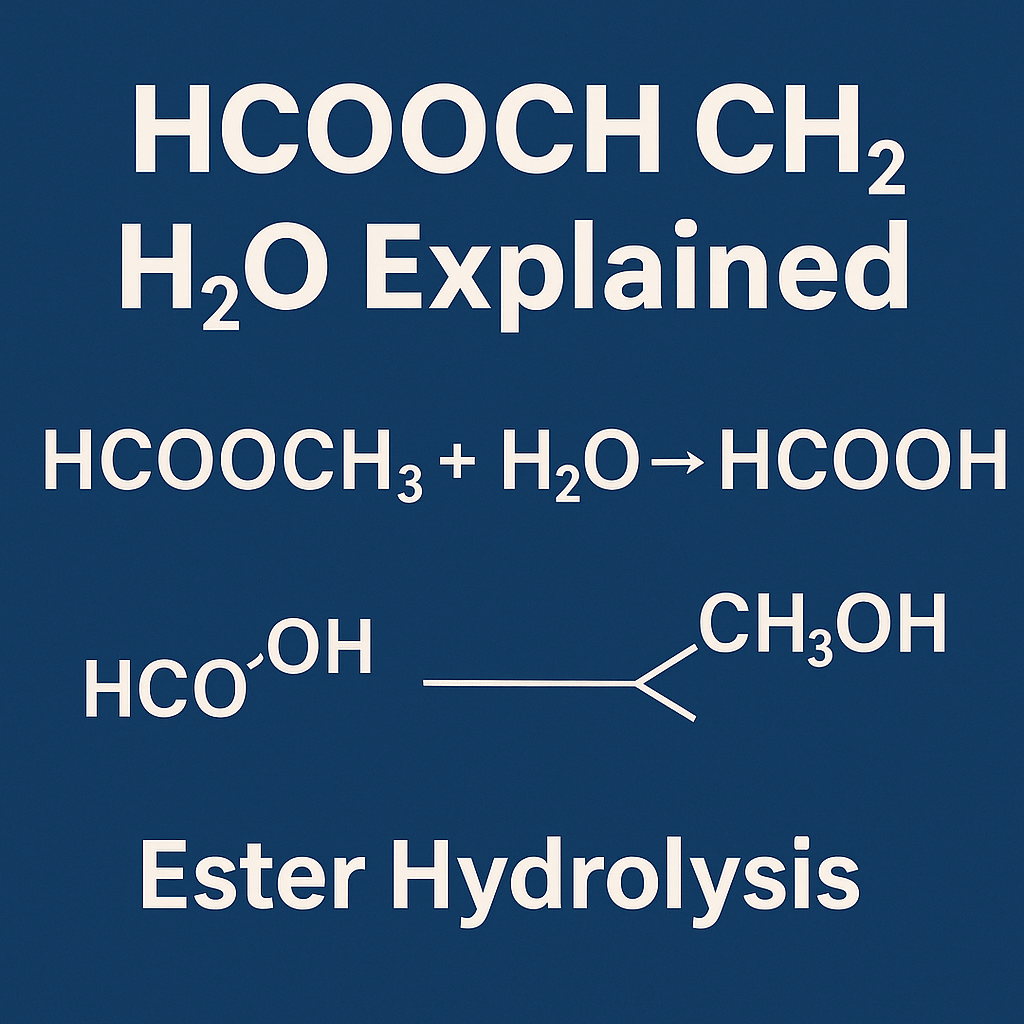
HCOOCH₃ = methyl formate (an ester).
-
H₂O = water.
-
Together they react and make:
-
HCOOH = formic acid.
-
CH₃OH = methanol.
-
This reaction is called ester hydrolysis. It is used in science lessons and also in industries to make useful chemicals.
What Does HCOOCH CH₂ H₂O Mean?
The formula looks confusing, but it simply points to methyl formate reacting with water.
Balanced equation:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
Reactants and Products
| Symbol | Name | Type |
|---|---|---|
| HCOOCH₃ | Methyl formate | Ester (reactant) |
| H₂O | Water | Reactant |
| HCOOH | Formic acid | Product (acid) |
| CH₃OH | Methanol | Product (alcohol) |
Why This Reaction Is Important
Uses in Science
-
Shows how esters break down.
-
Simple teaching reaction in organic chemistry.
-
Helps students learn about mechanisms.
Uses in Industry
-
Makes formic acid – used in leather, rubber, food, and hydrogen storage.
-
Makes methanol – used as solvent, fuel, antifreeze, and raw material for many products.
Applications of Products hcooch ch2 h2o Explained
| Product | Main Uses |
|---|---|
| Formic acid | Leather, food preservative, hydrogen storage, textiles |
| Methanol | Fuel, solvent, plastics, antifreeze |
What Type of Reaction In hcooch ch2 h2o Explained?
-
This is an ester hydrolysis reaction.
-
Hydrolysis means “breaking with water.”
-
The ester (methyl formate) reacts with water.
-
The result is an acid and an alcohol.
It is a basic example used in organic chemistry classes.
hcooch ch2 h2o Explained Acid-Catalyzed Pathway
In the presence of an acid (like HCl or H₂SO₄), the reaction follows steps:
-
The ester gets protonated (activated by acid).
-
Water attacks the carbonyl carbon.
-
A temporary tetrahedral intermediate forms.
-
Bonds rearrange, methanol is released.
-
Formic acid is made.
Acid Pathway Steps
| Step | Action | Result |
|---|---|---|
| 1 | Protonation | Ester becomes active |
| 2 | Water attack | New bonds form |
| 3 | Intermediate | Temporary structure |
| 4 | Breakdown | Products appear (HCOOH + CH₃OH) |
Base-Catalyzed Pathway hcooch ch2 h2o Explained
With a base like NaOH, the reaction is different:
-
Hydroxide ion (OH⁻) attacks the ester carbonyl.
-
Intermediate forms.
-
Bond breaks, methanol leaves.
-
Formate anion takes a proton and becomes formic acid.
Base Pathway Steps hcooch ch2 h2o Explained
| Step | Action | Result |
|---|---|---|
| 1 | OH⁻ attack | Carbonyl activated |
| 2 | Intermediate | Formed |
| 3 | Breakdown | Methanol released |
| 4 | Protonation | Formic acid formed |
Reaction Conditions hcooch ch2 h2o Explained
The speed of this reaction depends on several factors:
-
Acid catalyst – speeds it up.
-
Base catalyst – also speeds it up, but with a different path.
-
Excess water – pushes equilibrium to products.
-
Heat – increases reaction speed.
hcooch ch2 h2o Explained Conditions
| Factor | Effect |
|---|---|
| Acid catalyst | Faster, common in teaching |
| Base catalyst | Faster, alternate path |
| Extra water | More products formed |
| Heat | Higher reaction speed |
Kinetics and Equilibrium hcooch ch2 h2o Explained
-
Without catalyst → very slow.
-
With acid or base → much faster.
-
Reaction reaches equilibrium between reactants and products.
-
Extra water drives reaction to completion (Le Châtelier principle).
Real Uses and Applications hcooch ch2 h2o Explained
In Classrooms
-
Demonstrates ester hydrolysis.
-
Teaches step-by-step mechanisms.
-
Simple enough for beginners.
In Industry
-
Produces formic acid for many uses.
-
Produces methanol as an important chemical.
-
Part of larger chemical cycles using carbon monoxide and methanol.
Misconceptions to Avoid
-
Formula confusion – It is methyl formate (HCOOCH₃), not “HCOOCH CH₂.”
-
Mixing vs reaction – It is not simple mixing; bonds actually break.
-
Catalyst role – Without acid or base, it is too slow.
-
Not combustion – It is hydrolysis, not burning.
Practical Examples
-
Lab experiment – Students add acid to ester + water and watch products form.
-
Industrial plant – Hydrolysis is used to produce formic acid.
-
Research – Used in studies on hydrogen energy storage.
Consumer and Industrial Impact hcooch ch2 h2o Explained
For Consumers
-
Methanol appears in fuel, cleaners, and antifreeze.
-
Formic acid appears in food preservatives and textiles.
For Industry
-
Hydrolysis of methyl formate is part of large-scale chemical production.
-
Links organic reactions to real-life manufacturing.
Glossary
-
Ester – a compound formed from acid + alcohol.
-
Hydrolysis – breaking a bond using water.
-
Catalyst – speeds up a reaction but is not used up.
-
Intermediate – temporary structure in reaction.
-
Equilibrium – balance between reactants and products.
FAQs
What does HCOOCH CH₂ H₂O mean?
It is shorthand for the hydrolysis of methyl formate (HCOOCH₃) with water (H₂O). The products are formic acid (HCOOH) and methanol (CH₃OH).
What type of reaction is hcooch ch2 h2o Explained?
It is an ester hydrolysis reaction, where an ester reacts with water to form an acid and an alcohol.
What are the products of this reaction?
The products are formic acid (HCOOH) and methanol (CH₃OH).
Is this reaction fast?
No, it is slow on its own. It needs a catalyst like acid or base to become fast.
Conclusion
The formula HCOOCH CH₂ H₂O is a shorthand for the hydrolysis of methyl formate with water. The products are formic acid and methanol.
This reaction is a key example of ester hydrolysis. It is simple enough for teaching, but also important in industries where formic acid and methanol are needed.
In 2025, this reaction is not only a learning tool but also a practical process that connects classroom chemistry with real-world applications.